Calcium Stearate Sodium Stearate Zinc Stearate Hydrotalcite 1000 ppm Acid Acceptor added Polypropylene 25 MF nominal powder 500 ppm phenolic AO700 ppm secondary phosphorus. For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C.
Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html
Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate.

. Dibasic zinc stearate Zinc distearate Zinc salt of stearic acid CAS No. Water and zinc stearate were formed. Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin.
Zinc stearate has different ratios of palmitic and stearic acids. TABLE I FATTY ACIDS LENGTH LINEAR MELTPOINTC Lauric C12H24O2 saturated 44 Myristic C14H28O2 saturated 54. These applications exploit its non-stick properties.
Eyes skin respiratory system NIOSH 2022. The technology comprises the following steps. By monitoring features in the infrared spectra that are characteristic of the global conformation of the hydrocarbon chain it is shown that the double.
Water and zinc stearate were formed. The reaction was NaSt and zinc sulfate. From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and.
This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol. At 600 C no acid soap was detected. The products were characterized by XRD TG.
As discovered in the early days of v. For example the zinc stearate will melt during molding and be absorbed into the compound without leaving discoloration or defects on the surface of the final molded rubber part. Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging.
Influence of Calcium Monostearate on Recrystallization of PP 110 112 114 116 118 120 122 124 DSC Peak No AOMST AONo MST. It is a white powder and is insoluble in water. NMR and GC-MS measurements on methyl-esterified products from zinc stearate were used to evaluate each.
At the same time it also has the function of vulcanization activator and softener in rubber. Zinc stearate is high in preparation yield good in color and cluster low. For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C.
Reaction between ZnO and stearic acid forming zinc stearate and the molar masses of each chemical substance in the reaction. It is an activator for accelerated rubber sulfur vulcanization. The reaction between the two would produce zinc stearate and water.
The aqueous emulsion of zinc stearate is called zinc stearate. Zinc stearate and lead stearate were synthesized using PbO ZnO and stearate acid as the reactants. From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide.
Rubber polyurethane polyester processing system powder metallurgy. ZINC STEARATE 201 ZINC STEARATE 201ND and ZINC STEARATE 325 are fusion reaction zinc oxide and fatty acid tallow based zinc stearates suitable for FDA CFR 21 indirect food applications. Three reaction parameters including temperature time and amount of HCl per zinc stearate were varied to optimize the acid-catalyzed esterification reaction of zinc stearate while addition of toluene was regarded as an essential parameter.
Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following. Inhalation ingestion skin andor eye contact. In cosmetics zinc stearate is a lubricant and thickening agent used to improve texture.
The influence of a double bond in the middle of an otherwise flexible hydrocarbon chain on the melting of such assemblies has been investigated by comparing the melting behavior of zinc stearate and zinc oleate. DOT ID Guide. The reagents in Raw Material Characterization.
The reaction between the two would produce zinc stearate and water. These three products are all manufactured using the same raw materials and same base process but finished differently to provide a range of. Na 909 C 6614 O 1445 also carried out at 10 excess sodium stearate and 10 wt and Zn 3927 S 1539 O 4068 wt values excess zinc sulfate.
2 zinc oxide and a catalyst are added stepwise condensing dewatering and salt forming are conducted and zinc stearate is synthesized. When reacting stearic acid and zinc hydroxide hydrochloric acid is. EDX analysis of eq 1 were used in equivalent amounts.
As an anti-adhesive and anti-tacking agent Zinc Stearate reduces adhesion between rubber surfaces and in rubber products. It is widely used as a release agent for the production of many kinds of objects. Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn.
A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant. Irritation eyes skin upper respiratory system. Product to use on the surface of the uncured rubber.
It can also act as a lubricant mold release agent and dusting agent for rubber preventing the rubber from sticking to the mold as well as to. Excerpt from NIOSH Pocket Guide for Zinc stearate.

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Pdf Zinc Stearate Production By Precipitation And Fusion Processes Semantic Scholar

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram
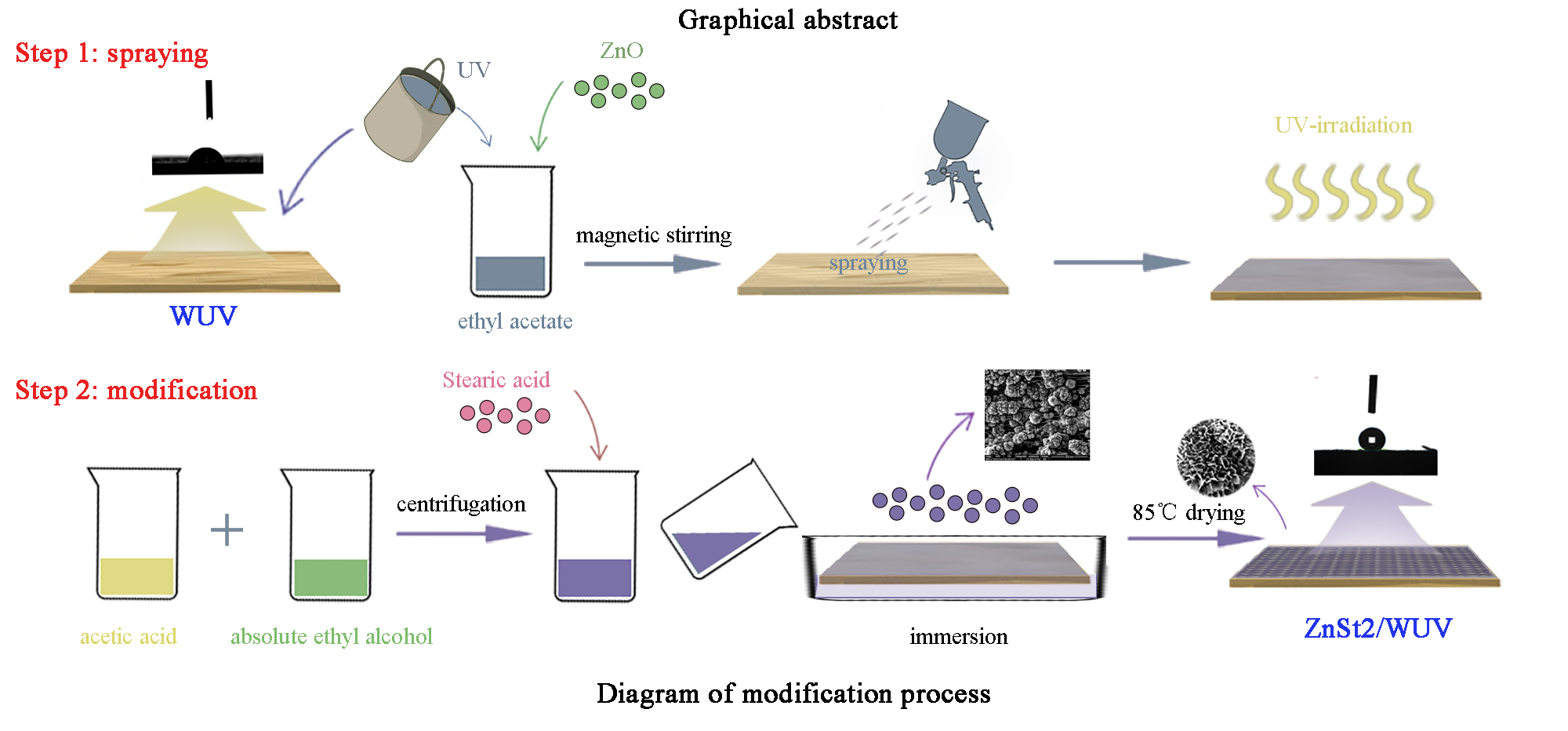
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Measured Characteristics Of The Prepared Zinc Stearate Download Table
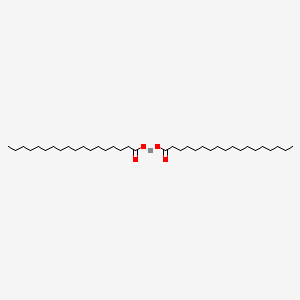
Zinc Stearate C36h70o4zn Pubchem

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Zinc Stearate Technical Grade 557 05 1

Tga Dsc Curves Of Zinc Stearate Reveal A Relative Thermal Equilibrium Download Scientific Diagram

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram
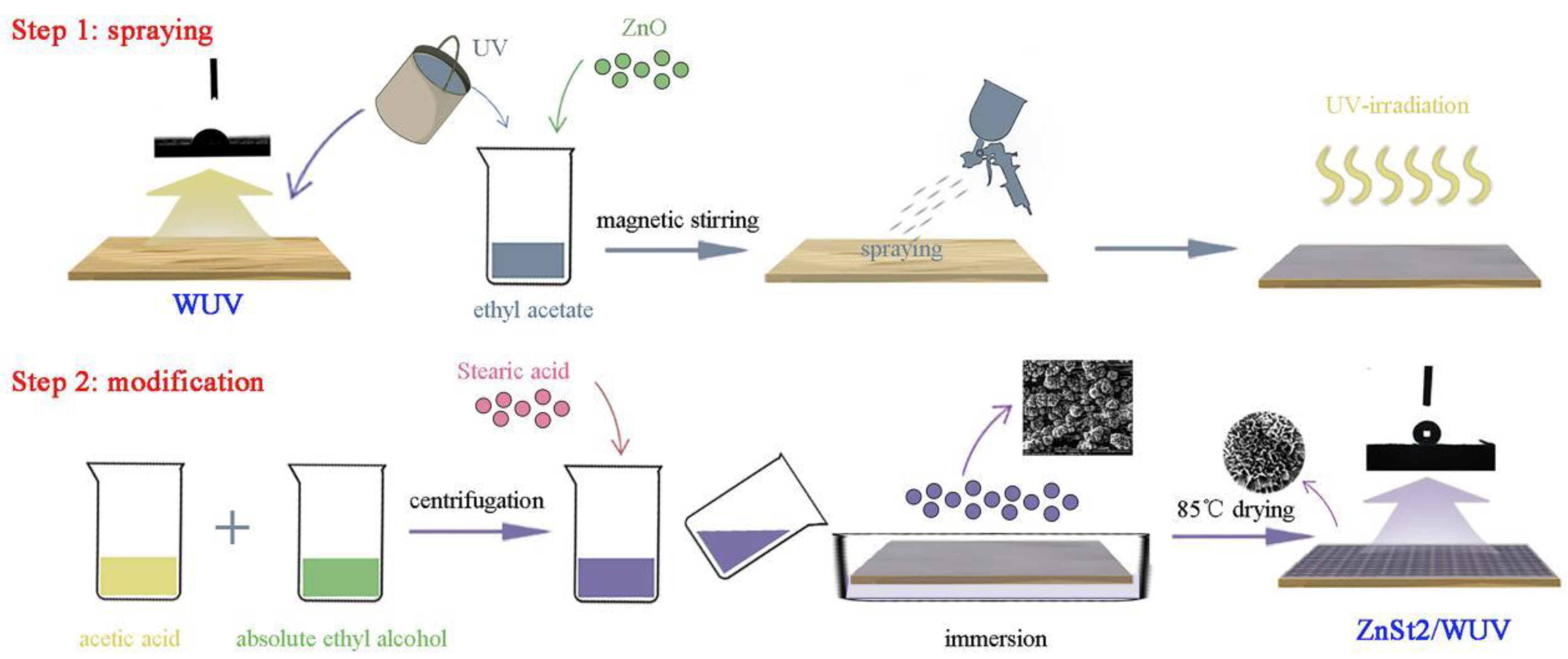
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect

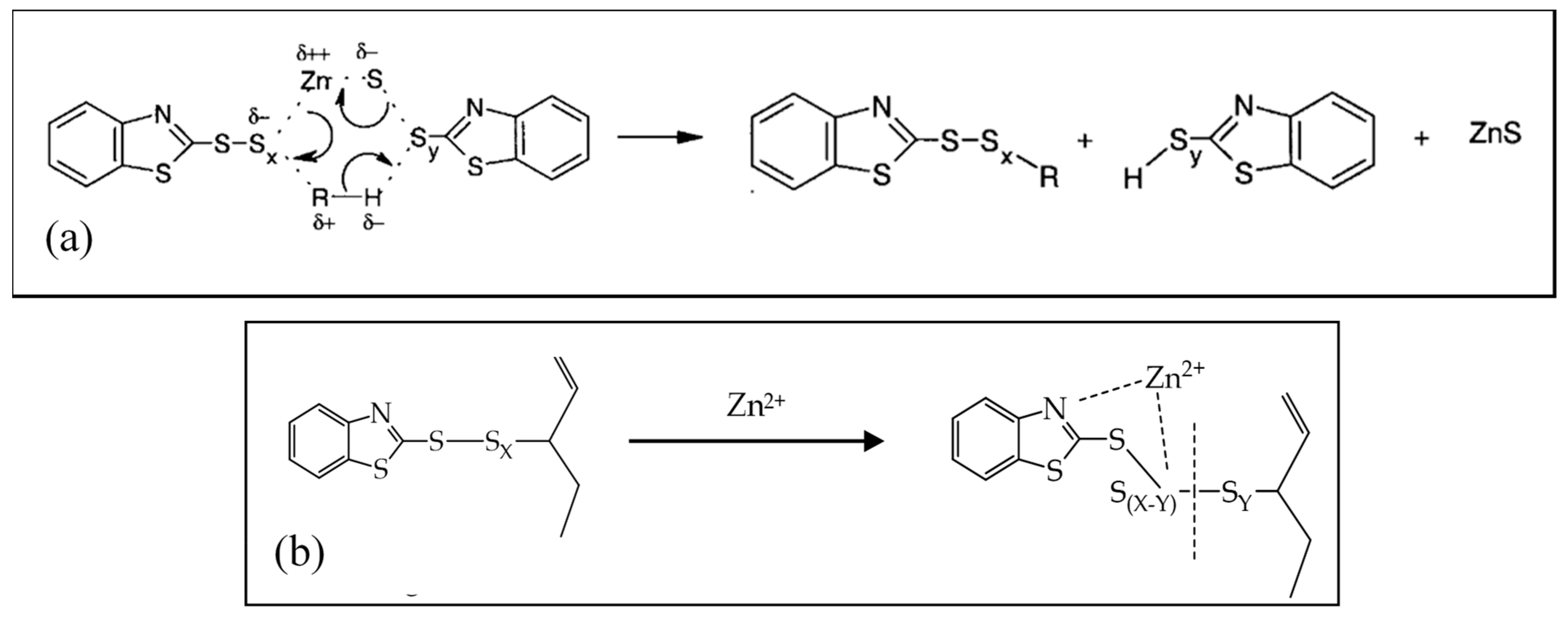
Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Ion Exchange Between Silanol Groups And Zinc Stearate On Silica Surface 28 Download Scientific Diagram

